Abstract

Protein phosphatase Mg2+/Mn2+-dependent 1D (PPM1D) gene (17q23) cooperates with TP53 in regulating DNA damage response (DDR). PPM1DMT are frequently encountered in clonal hematopoiesis of indeterminate potential (CHIP) and in therapy-related myeloid neoplasms (t-MN). Most typically lesions lead to C-terminal loss leading to truncation with paradoxical gain-of-function amplification of PPM1D effects in terms of inactivation of TP53 and are thus functionally synergistic with TP53 deficiency. Here, we investigated the clonal architecture and clinical implications of PPM1DMT in the context of TP53MT MN.
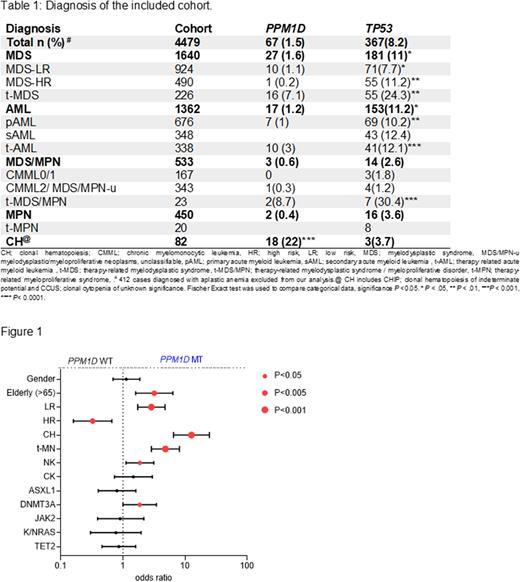
A cohort of 4479 patients (pts) screened for the presence of PPM1DMT and TP53MT was analyzed in terms of clinical phenotype, cytogenetics, clonal hierarchy and outcomes (see Table). Overall, 67 (1.5%) pts harbored PPM1DMT and 367 (8%) TP53MT. PPM1DMT (n=70) were truncating, of which 57% were frameshift and 43% were stop codons, distributed across exon 6. No biallelic PPM1DMT case was found. When we studied PPM1D expression in wild type, mRNA levels were elevated in AML (59%; P<.0001, but not in MDS), with high risk AML having higher expression PPM1D levels compared to favorable cases (59% vs. 46%, P=.08). PPM1DMT cases were older than those with TP53MT [median 74yrs (range, 39-93) vs. 71yrs (14-95), P=.02]. PPM1DMT were significantly enriched within pts with CH (including CHIP and CCUS) compared to TP53MT (27 vs. 1%, P=.0001) while TP53MT were more frequently encountered in MN (71 vs. 31%, P=.0001). Review of the medical records of PPM1DMT and TP53MT cases revealed history of malignancy was ascertained in 69% of PPM1DMTvs. 30% in TP53MT; P<.0001, of which 30% had hematological malignancies (HM) and 7.5% were breast cancer (BC) survivors. Of note is that 9% PPMD1MT cases had autoimmune disorders. Overall, 37% received only chemotherapy, 20% radiotherapy only, 22% both chemo/radiotherapy and 13% immunosuppression. A total of 14% of TP53MT carriers had a prior history of HM and 5% had BC history. When compared to PPM1DMT, a higher fraction (58%; P=.005) received chemotherapy, whereas similar rates were noted for radiotherapy (14%), chemo/radiotherapy combination (17%) and immunosuppression (11%).
PPM1DMT were also more commonly found in t-MN than TP53MT (42 vs. 28%, P=.05). Overall, 37% of PPM1DMT showed a normal karyotype (NK) compared to 14% of TP53MT (P=.0003). Indeed, up to 61% of TP53MT had complex karyotypes (CK vs. 15% in PPM1DMT, P=.0001). Most common genes associated with PPM1DMTvs.TP53MT were DNMT3A (21 vs. 10%; P=.05), TET2 (19 vs. 11%; P=.2), ASXL1 (15 vs. 10%, P=.4), KRAS/NRAS (1 vs 7%, P=.06) and NF1 (1 vs. 7%, P=.03).
Analysis of clonal architecture showed that PPM1DMT were either dominant / codominant (46%; n=33) including PPM1DMT as a sole lesion(n=19); in these cases TP53MT was the most common 2nd/codominant hit (25%) followed by DNMT3A (12.5%, P=.04) and RUNX1 (12.5%, P=.04). Cases with dominant PPM1DMT were encountered more in t-MN (46 vs. 36%, P=.3) and CH (30 vs. 24%, P=.4) compared to subclonal PPM1DMT (n=34, 51%), which were subclonal to TET2MT (21%) followed by JAK2 (12%), DNMT3A (9%, P=.02) and TP53 (9%, P=.02). Notably, all deaths in PPM1DMT CH cases were not due to progression to MN. No significant difference was found in OS between ancestral and subclonal PPM1DMT carriers.
PPM1DMT were more likely encountered in older pts (OR=3.2; P=.001), MN-LR (OR=2.8, P<.0001), CH (OR=12.6, P<.0001), t-MN (OR=4.8, P<.0001), NK (OR=1.9, P=.01) and DNMT3AMT carriers (OR=1.86, P=.04) and less likely to occur in pts with MN-HR (OR=0.38, P=.002; see Figure)
Survival analysis showed no significant difference between PPM1DMT and PPM1DWT and between CH and non-CH PPM1DMT cases while there was a significant difference between PPM1DMTvs. TP53MT (102 vs.12mo., P<.0001).Of note is that 12 pts had both PPM1DMT and TP53MT (50% t-MN, of which 25% had a prior history of lymphoma). In such cases, PPM1DMT were mostly ancestral compared to TP53MT (67 vs. 33%; P<.0001).
Configurations of PPM1D in the clonal hierarchy and pairing with secondary or primary hits are consistent with their role in MN evolving from CH. Association with CH implies that some of the t-MN may be derived from pre-existing CH a theory which we are currently being testing in serial samples.
Disclosures
Patel:Apellis Pharmaceuticals: Consultancy, Other: Teaching and Speaking; Novartis Pharmaceuticals: Consultancy; Alexion: Consultancy, Other: Teaching and Speaking. Maciejewski:Apellis Pharmaceuticals: Consultancy; Alexion: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal